Radical Hysterectomy for Carcinoma of the Uterine Cervix
Authors
INTRODUCTION
Interest in developing a surgical cure for carcinoma of the uterine cervix predates the turn of the 20th century. Working independently, Clark,1 Rumpf,2 and Ries3 described the radical abdominal hysterectomy with lymph node dissection in the mid-1890s. In 1902, citing the unacceptable surgical mortality of the abdominal approach, Schauta advocated the radical vaginal hysterectomy.4 Wertheim criticized the vaginal procedure for its failure to include an assessment of the lymph nodes. Shortly thereafter, Wertheim published a series of cases describing his experience with the radical abdominal hysterectomy and selective pelvic lymph node sampling.5, 6, 7
In spite of these early attempts, radical pelvic surgery was not enthusiastically embraced owing to excessive morbidity and mortality; thus radiation remained the mainstay of treatment for cervical cancer during the first half of the 20th century. Nevertheless, some surgeons remained interested in refining the procedure. In 1934, Taussig reported a survival benefit when pelvic lymphadenectomy was added to standard radiotherapy.8 During the following decade, Meigs led a revival in radical surgery for carcinoma of the cervix. He modified the operation described by Wertheim to include more extensive removal of the parametria and a complete pelvic lymphadenectomy.9, 10, 11 Although complications, particularly fistulas, remained a significant obstacle to the widespread endorsement of the radical hysterectomy for the treatment of cervical cancer, the mortality associated with this procedure was virtually eliminated as antibiotics and blood transfusions became increasingly utilized.
GENERAL CONSIDERATIONS FOR RADICAL HYSTERECTOMY
Appropriate selection is critical when considering whether surgical therapy would be most beneficial to a patient with carcinoma of the cervix. Some patients are considered poor surgical candidates because of their comorbidities. Frequently cited medical contraindications to radical hysterectomy include cardiac, pulmonary, and renal diseases. Thromboembolism and coagulopathy should be considered as well. Conversely, certain situations lend themselves to primary surgical therapy. Complications associated with radiation may preclude its use in patients with pelvic adhesive or inflammatory disease, previous genitourinary or intestinal surgery, inflammatory bowel disease, and anatomic anomalies (e.g., pelvic kidney).
Many gynecologic oncologists favor nonoperative treatment in elderly patients. Sixty-five years of age is frequently cited as the limit for consideration of radical hysterectomy. However, some authors have found that the morbidity and survival of older patients is comparable with that of younger patients.12, 13, 14, 15 Thus, it seems prudent to determine whether a surgical approach is appropriate and safe based on an assessment of risk factors and comorbidities independent of chronologic age.
Obesity is frequently associated with medical comorbidities that preclude consideration of radical hysterectomy. Furthermore, concerns about the feasibility of performing adequate resection and the exposure of obese patients to intraoperative and postoperative complications have discouraged some surgeons from considering these patients as surgical candidates. Recently, this position has been reconsidered; several authors have pointed out that, in carefully selected obese patients, radical hysterectomy can be performed adequately, safely, and effectively, although operative time and blood loss may be greater.14, 16, 17, 18
Ovarian preservation is desirable in many instances. Indeed, this is one of the major advantages of surgical therapy in young patients who would otherwise require postoperative hormone replacement. Ovarian metastases are so rare (<1%) when squamous carcinoma is present that consideration of oophorectomy may be indicated only when there is gross spread to the adnexa or concomitant ovarian pathology or the patient is menopausal.19, 20, 21 In contrast, the risk of ovarian metastases has been reported to be as high as 19% for patients with stage I or II adenocarcinoma of the cervix, prompting some to recommend bilateral oophorectomy when this histologic diagnosis is known preoperatively.19, 22, 23 Importantly, care should be taken to reposition the ovaries outside the pelvis in the event that postoperative radiation is needed;24 ovarian failure can be reduced to as low as 17% when this procedure is combined with shielding during adjuvant radiotherapy.25, 26
Sexual function is an important consideration and should be discussed with all patients regardless of their age. Although the vagina is invariably shortened after a radical hysterectomy, vaginal length can be restored when sexual activity resumes. Moreover, the vaginal epithelium and caliber remain relatively unchanged with surgery; irreversible atrophy and stenosis of the vagina occur frequently with radiation, leading to sexual dysfunction in up to 78% of patients treated with radiotherapy.27, 28 Nevertheless, some surgically treated patients will have sexual dysfunction related to neurovascular damage or alterations in body image and self-esteem.29, 30, 31 In a longitudinal study of 173 patients treated with radical hysterectomy, a persistent lack of sexual interest and vaginal lubrication were self-reported over 2 years of follow-up. The majority of other sexual problems, such as anorgasmia and dysparunia, improved over time.32 Despite the potential for sexual dysfunction after surgery, cervical cancer patients treated with radical hysterectomy have significantly better quality of life and sexual functioning compared to matched controls treated with radiotherapy.33
Diagnostic evaluation occasionally influences treatment planning. The International Federation of Gynecology and Obstetrics (FIGO) advocates clinical staging of cervical carcinoma based on physical examination findings and select, widely available diagnostic studies, such as hysteroscopy, cystoscopy, proctoscopy, intravenous urography, and X-ray examination of the lungs and skeleton. However, it is well known that clinical staging is frequently inaccurate; a substantial number of patients who undergo surgical treatment are found to have metastases that upstage their disease34, 35, 36, 37 or benign findings that downstage their disease.35 Improved imaging studies such as high-resolution computed tomography (CT),38, 39, 40, 41 magnetic resonance imaging (MRI),40, 41, 42 lymphangiography,43, 44 and positron emission tomography45, 46 may reveal extrauterine disease that cannot be cured with standard surgical techniques. In a study comparing clinical exam with MRI and CT imaging in the evaluation of cervical cancer, MRI was superior to CT for evaluating uterine body involvement and measuring tumor size, but no method accurately evaluated cervical stromal involvement.47 These radiographic studies are imperfect because they fail to detect micrometastases that may impart a poor prognosis for surgical patients. Additionally, some clinicians advocate pretreatment surgical staging, typically using an extraperitoneal approach.48, 49, 50 Although the actual survival benefit conferred by such procedures may be limited,51 it is a useful adjunct in investigational settings.
Another diagnostic technique intended to reduce the morbidity of a full lymph node dissection is sentinel node evaluation. This approach has been successfully utilized in other cancers, particularly breast cancer and malignant melanoma. The sentinel node is the first node to receive lymphatic drainage from the cancer. If the sentinel node is negative for metastases, then more distant nodes are also likely to be negative and would not need to be resected. The nodal status in women with cervical cancer is vitally important, both for prognosis and guiding adjuvant therapy. An analysis of studies totaling over 800 patients reveals a sensitivity of 92% and a negative predictive value of 97%.52 In other words, 8% of patients undergoing sentinel node detection will be falsely negative, and if the sentinel node is negative, the rest of the nodal basin will be negative 97% of the time. Various techniques for lymphatic mapping have been employed, including vital blue dye,53 radiocolloid,54 or a combination.55 A combination technique appears to yield the best results.56 However, the addition of preoperative lymphoscintigraphy does not seem to have an advantage over intraoperative lymphatic mapping.57 Currently, sentinel lymph node evaluation in early-stage cervical cancer continues to be investigational. Improving the false-negative rates and evaluating the long learning curve are challenges that must be addressed before this technique gains widespread acceptance.
TYPES OF RADICAL ABDOMINAL HYSTERECTOMY
In 1974, Piver and colleagues proposed a new classification system to clarify the ambiguities of the existing terminology used to differentiate types of abdominal hysterectomies.58 This system describes the extent of dissection of hysterectomies performed for various stages of cervical cancer, thereby identifying potential surgical complications. The following is a limited description of each procedure; a complete discourse on the technique for each type of hysterectomy can be found in several texts and atlases.59, 60, 61, 62, 63, 64
The type 1 hysterectomy is commonly referred to as the extrafascial, or simple, hysterectomy. This hysterectomy removes the cervix along with the uterine corpus, but does not require mobilization of the ureter or removal of a significant amount of the parametria.
The type 2 (modified) radical hysterectomy, or Wertheim operation, requires more extensive dissection than the extrafascial hysterectomy. The central portion of the parametrial tissues is removed while minimizing disruption to the ureteral and vesical vasculature. Thus, the medial half of the uterosacral ligaments and the cardinal ligaments are removed as the uterine artery is ligated just medial to the point at which it crosses the ureter. Removal of a 1–2-cm portion of the upper vagina and pelvic and para-aortic lymphadenectomy are nearly always performed in conjunction with a type 2 radical hysterectomy.
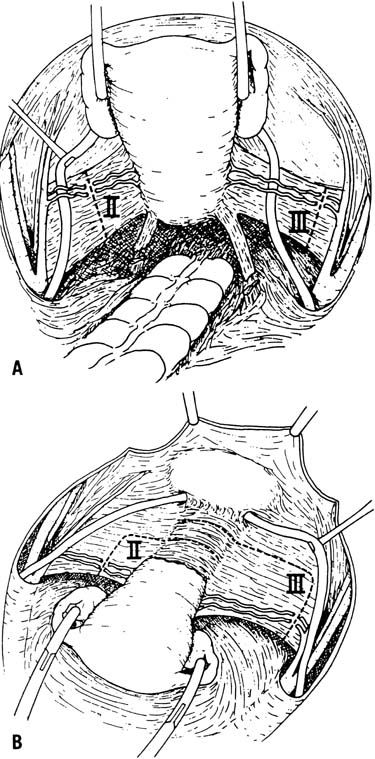
The type 3 radical hysterectomy, originally described by Meigs, aims to remove as much parametrial tissue as possible. The uterosacral ligaments are transected near their origin from the sacrum. The cardinal ligaments are excised as widely as possible after the uterine artery is ligated at its origin where it branches off the hypogastric artery. Care should be taken to preserve the superior vesical artery. Removal of a 2–3-cm portion of the upper vagina and a pelvic and para-aortic lymphadenectomy is performed in conjunction with a type 3 radical hysterectomy (Fig. 1).
|
The type 4 hysterectomy is seldom used, but represents an extended radical hysterectomy. It involves complete dissection of the ureter from the vesicouterine ligament, sacrifice of the superior vesical artery, and removal of up to 75% of the vagina. The type 5 hysterectomy also has limited applicability. This procedure involves resection of involved portions of the bladder or distal ureter with subsequent ureteral reimplantation. Occasionally, it substitutes for an anterior exenteration.
MINIMALLY INVASIVE SURGICAL APPROACHES TO RADICAL HYSTERECTOMY
Advantages of laparoscopic surgery compared to open laparotomy have been well recognized. Compared to abdominal radical hysterectomies, total laparoscopic radical hysterectomies result in reduced operative blood loss, postoperative wound infections, and length of hospital stay.65 Similarly, a meta-analysis of prospective trials studying simple hysterectomies for benign disease found that compared to open laparotomy, laparoscopic hysterectomies result in less pain, shorter hospital stays (by an average of 2 days), decreased wound infections (relative risk decreased by 80%), and quicker return to normal activity (2 weeks sooner).66
The da Vinci robotic system (Intuitive Surgical Corporation, Sunnyvale, CA) has been increasingly utilized in gynecologic surgery. Compared to traditional laparoscopy, the da Vinci robotic system provides improved three-dimensional vision and increased range and precision of movement with articulating instruments, facilitating precise tissue dissection and surgical knot-tying. In one series, robotic radical hysterectomy had similar operating times compared to laparotomy with significantly reduced blood loss and length of hospital stay.67 Not surprisingly, length of surgery is dependent upon operator experience.68 The robotically assisted surgical approach is a safe and feasible alternative to abdominal radical hysterectomy.
SPECIFIC INDICATIONS FOR RADICAL HYSTERECTOMY AND OUTCOMES
FIGO defines microinvasive carcinoma of the cervix primarily by depth of stromal invasion. Stage Ia1 disease is limited to those cervical cancers with 3 mm or less of invasion. Lesions with invasion to a depth of greater than 3 mm but no more than 5 mm are considered stage Ia2. Notably, this definition does not address lymphovascular space involvement. Significant controversy surrounds the prognostic significance of this histologic finding and whether it should guide treatment planning if it is known to exist.69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86 Whether or not lymphovascular space involvement is present, surgical therapy for stage Ia disease is curative for nearly all patients.61, 83, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99
Extrafascial hysterectomy is generally considered adequate treatment for stage Ia1 cervical cancer. Ostor's review of the literature reveals that in spite of a 3.7% risk of lymphovascular space involvement, the risk of recurrence and lymph node metastases is approximately 1% and the risk of cancer death is only 0.2%.95 Nonetheless, some gynecologic oncologists advocate the same treatment for stage Ia1 cervical cancer with extensive lymphovascular space involvement as for stage Ia2 disease. If fertility is to be preserved, a cervical conization may suffice, provided the surgical margins and postconization endocervical curettage are negative for carcinoma and dysplasia.91, 97
Stage Ia2 cervical cancer is usually treated with a type 2 modified radical hysterectomy with pelvic lymphadenectomy. Hacker summarized the data on invasive lesions of 3–5-mm depth and found that nodal metastases occur in 7.3%, recurrences occur in 3.1%, and cancer death occurs in 2.3%.61 Lymphovascular involvement may be seen in as many as 18.4% of these patients,95 thus reinforcing the questionable significance of this finding in microinvasive disease. When future childbearing is desired, cervical conization91, 97 or radical trachelectomy99 may be combined with extraperitoneal or laparoscopic lymphadenectomy.
Macroscopic early-stage tumors are also treated successfully with surgery. The type 3 radical hysterectomy with pelvic and para-aortic lymphadenectomy is used for patients with stage Ib and IIa cervical cancer. Numerous authors have found that cure rates approach 85–90% and are comparable with the outcomes of patients treated with primary radiotherapy.100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124
Recently, some have proposed that in select patients with stage Ib1 cervical cancer, more conservative treatment may be appropriate in order to preserve future fertility or improve quality of life. Several studies have shown that some patients with Ib1 cervical cancer may have been successfully cured with simple conization and would have not required radical surgery. Covens et al. showed that the risk for positive parametria is only 0.6% in patients with negative lymph nodes, tumor <2 cm in size and <1.0 cm depth of invasion;125 these results have since been borne out in additional studies126, 127 indicating that more conservative treatment may be appropriate in certain patients. Early studies by Rob and Ditto demonstrated, albeit with small numbers, that conservative surgery (simple trachelectomy and simple conization with lymph node dissection, respectively) is feasible in the treatment of Ia2 and Ib1 cervical cancers.128, 129 With this in mind, a recent study was conducted by Maneo et al. that evaluated outcomes in 36 women with Ib1 cervical cancer who were treated with simple conization and lymph node dissection. None of the 36 patients had positive lymph nodes, and after median follow-up of 66 months, only one of the 36 patients had recurred. Five patients underwent later simple hysterectomy, one of whom demonstrated residual microinvasive disease in the hysterectomy specimen. Fourteen live births have occurred in this cohort, indicating a favorable obstetric outcome.130 Similarly, the Gynecologic Oncology Group is currently designing a study to evaluate quality of life, sexual function, and reproductive outcomes in women with 1A1–1B1 cervical cancer who are treated with either extrafascial hysterectomy or cervical conization with pelvic lymphadenectomy.
Although these studies show that a majority of patients with stage Ib carcinoma of the cervix are cured with radical hysterectomy, and perhaps more conservative surgical approaches, it became evident that patients with large tumors had significantly worse outcomes. It is now well established that primary surgical therapy for bulky (tumor diameter >4 cm) or barrel-shaped stage Ib disease is associated with a greater risk of lymph node metastases, local and distant recurrence, and cancer death.120, 131, 132, 133, 134, 135, 136, 137 Unfortunately, stage Ib patients treated with primary radiotherapy also tend to have poorer outcomes as tumor size increases138, 139, 140, 141 because the high doses of radiation required to sterilize large tumors exceed the amount of radiation tolerated by normal tissues.
FIGO acknowledged this survival difference by modifying the staging system. There is a distinction between smaller Ib1 lesions and larger stage Ib2 tumors. However, in a review of patients with stage Ib cervical cancer who had been treated with radical hysterectomy, Finan demonstrated that stage was not an independent predictor of poor outcomes. Rather, he showed that stage imparted a worse prognosis vis-à-vis nodal metastases, and that patients with stage Ib2, node-negative disease had significantly better survival than stage Ib2, node-positive disease.142 Likewise, Rutledge and colleagues compared stage Ib1 and Ib2 cervical cancers treated by radical hysterectomy, and found that tumor size failed to predict nodal metastases or recurrence when Ib tumors were stratified according to lymphovascular space invasion, depth of invasion, and parametrial involvement.143 Furthermore, the Gynecologic Oncology Group (GOG) and others have studied patients with “intermediate-risk” stage Ib tumors (including stage Ib2) treated with primary radical hysterectomy and have yet to demonstrate a survival benefit with adjuvant radiation in the absence of nodal metastases or positive surgical margins, although postoperative pelvic irradiation does reduce the risk of recurrence and prolongs progression-free survival for these patients.144, 145, 146
While 1B1 cervical cancer has potential to be cured with conservative surgical management, the optimal treatment for stage Ib2 cancer of the cervix remains controversial. Currently, the treatment options include radical hysterectomy with pelvic lymph node dissection and para-aortic lymph node sampling, brachytherapy plus pelvic radiation with concurrent cisplatin-based chemotherapy, or cisplatin-based chemoradiation with brachytherapy followed by adjuvant hysterectomy.147, 148, 149, 150
Several cost-analysis studies have suggested that radical hysterectomy may be the most cost-effective treatment strategy for stage Ib2 cervical cancer.151, 152 Although treatment with neoadjuvant chemotherapy prior to radical hysterectomy has been considered in bulky stage Ib cervical cancer, a recent phase III randomized trial from the Gynecologic Oncology Group failed to demonstrate any benefit from pretreatment with cisplatin and vincristine prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy.153
In select situations, recurrent or advanced cervical cancer can be managed surgically. Five-year survival in patients with small pelvic recurrences (<2 cm) confined to the cervix treated with radical hysterectomy has been reported to be as high as 84–90%;154, 155 however, the incidence of major postoperative morbidity is 31–44%.154, 155, 156 Conservative surgical treatment of larger recurrences is ill advised, because as tumor size increases, the overall survival decreases and the incidence of surgical complications rises dramatically.156
COMPLICATIONS OF RADICAL HYSTERECTOMY
Radical hysterectomy can be accomplished with acceptable morbidity (<5%) in the hands of an experienced surgeon. Nevertheless, complications do occur. Predisposing factors include previous pelvic surgery or radiation, endometriosis, pelvic inflammatory disease, anatomic anomalies, obesity, and pregnancy.
Intraoperatively, the most common complication of radical hysterectomy is hemorrhage; the range of reported average blood loss is from 600 mL to 1900 mL.14, 157, 158, 159 Injured vessels can be repaired with hemoclips or suture ligatures, although hypogastric artery ligation is sometimes required to control hemorrhage. Ureteral injury is recognized intraoperatively in less than 1% of cases,160, 161 however, it may occur more frequently and go unnoticed. The use of intravenous indigo carmine may help identify the site of injury. When an injured ureter needs repair, techniques such as ureteroneocystostomy, ureteroureterostomy, stenting, and retroperitoneal drainage may be required. Bladder and bowel injuries also occur, particularly when electrocautery is used inappropriately as a substitute for sharp dissection. Typically, cystotomies or enterotomies can be repaired with a two-layer closure. However, proper repair of some injuries necessitates more involved procedures such as ureteral stenting for trigone injuries or colostomy for extensive colonic injuries.
Complications that arise during the postoperative period include both early and late complications. Early complications, or those occurring within the first 30 postoperative days, may vary.158, 162, 163 Infectious and febrile morbidity is the most common postoperative complication. Prophylactic use of broad-spectrum antibiotics reduces the frequency of this complication.164 Postoperative bleeding may require reoperation; however, most cases are self-limited and can be treated conservatively with close observation for hemodynamic instability and blood transfusions. Clinically significant thromboembolic complications occur in approximately 5% of cases.158, 162, 163, 165, 166, 167, 168 Early diagnosis requires a high index of suspicion because clinical findings are frequently subtle or nonspecific. Prolonged ileus or intestinal obstruction occurs occasionally, but both conditions usually resolve with conservative management. Postoperative mortality in this era has been reduced to less than 1%.169
Voiding dysfunction in the immediate postoperative period is nearly universal; denervation of the bladder during the operation results in transient hypertonia that is gradually replaced by hypotonia.170, 171, 172, 173, 174, 175, 176, 177, 178 Bladder drainage can be achieved with suprapubic catheterization, intermittent self-catheterization, or indwelling urethral catheterization. For most patients, the ability to void returns within 2–3 weeks; however, voiding dysfunction may persist in approximately 5% of patients.158, 162, 163, 179, 180, 181 In addition, a substantial number of patients develop persistent urinary incontinence postoperatively. Pure stress urinary incontinence, urge incontinence, and mixed incontinence have been reported, although the incidence and nature of preoperative voiding dysfunction in these patients is unknown.172, 177, 179, 182, 183 Interestingly, in a study of 20 patients undergoing extensive urodynamic testing both before and after radical hysterectomy, only 20% revealed normal urodynamic findings prior to surgery.184
Historically, urinary tract fistulas were among the most dreaded postoperative complications. Interruption and mobilization of the vasculature of the bladder and ureters predisposes to ischemia that lends itself to fistula formation. The need for postoperative radiation therapy worsens this problem. Fortunately, the incidence of this troublesome complication is now quite low, occurring in less than 2% of cases.112, 113, 117, 169, 185 The diagnosis can be made by sequential inspection of a vaginal tampon after intravesical instillation of methylene blue followed by intravenous indigo carmine to determine whether a vesicovaginal or ureterovaginal fistula exists. Alternatively, an intravenous pyelogram or computed tomography may locate the fistula. Vesicovaginal fistulas, particularly those that are small, may heal spontaneously with prolonged bladder drainage; however, larger defects and those that fail to heal with conservative management need to be repaired surgically. Ureterovaginal fistulas require stenting; if a retrograde stent cannot be passed, percutaneous nephrostomy with anterograde stenting is required.
Other late postoperative complications (arising after 30 postoperative days) may be seen as well. Fortunately, they occur less frequently. Lymphedema develops insidiously over time, making its true incidence difficult to determine. It occurs more frequently when pelvic lymphadenectomy is followed by radiation or groin node dissection. Similarly, lymphocyst formation can occur as a result of extensive pelvic lymphadenectomy. The reported incidence is only 2–3%;169, 186, 187 however, many are asymptomatic and may go undetected. In the event that a lymphocyst causes ureteral obstruction or presents as a pelvic mass, percutaneous drainage or reoperation with marsupialization may be necessary. Sexual dysfunction and surgical menopause, which were previously discussed, should not be forgotten because quality of life will be important to the many survivors of early cervical cancer treated with radical hysterectomy.
SPECIAL CONSIDERATIONS
In the event that invasive cancer of the cervical stump is diagnosed in a patient with a prior subtotal hysterectomy, the staging and treatment options are essentially the same as for a patient with uterus in situ. There is no reason to believe that cancer of the cervical stump carries an inherently worse prognosis.187, 188 Early-stage disease can be effectively treated with radical trachelectomy; however, adhesions from the previous surgery increase the technical difficulty of the procedure and may prevent the attainment of adequate surgical margins.189, 190, 191, 192 If radiation is to be used, difficulty with dosing may occur because the cervical stump may be too short to properly support a tandem.
Rarely, pathologic evaluation of a simple hysterectomy specimen will reveal unsuspected invasive cervical cancer. When this occurs, further treatment is needed because of the possibility of residual disease in the vaginal cuff, parametria, or lymph nodes. Radiotherapy has been used successfully in these cases; however, the combination of surgery and radiation increases the likelihood that postoperative complications will arise. A reasonable alternative in selected patients is reoperation; radical parametrectomy with upper vaginectomy and lymphadenectomy may be curative.193, 194 Unfortunately, some patients who undergo radical reoperation will still require postoperative chemoradiation for positive surgical margins or lymph node metastases. Furthermore, this is a technically difficult procedure that usually requires a cystotomy to complete and subjects the patient to a second major surgery relatively soon after the initial procedure.
Interest in the radical vaginal hysterectomy, or Schauta procedure, has recently increased as laparoscopic surgery is incorporated into gynecologic oncology. Typically, the radical vaginal hysterectomy is preceded by an extraperitoneal laparoscopic lymphadenectomy. Alternatively, a laparoscope-assisted radical vaginal hysterectomy can be performed along with the laparoscopic lymphadenectomy. Descriptions of these procedures are available.195, 196, 197
The treatment of cervical cancer during pregnancy poses several challenges. Diagnosis may be delayed because the possibility of cancer is overlooked in young patients and because the most common presenting symptoms, vaginal bleeding or discharge, are attributed to pregnancy-related causes. Physiologic softening of the cervix and parametria may lead to errors in staging. The significance of mode of delivery has been questioned; however, most clinicians recommend a classic cesarean section; vaginal delivery may predispose to hemorrhage or cervical laceration or may spread to the lower genital tract including the episiotomy site.198, 197, 199, 200, 201, 202 Whether to proceed with immediate treatment or await fetal viability is subject to conflicting concerns for fetal and maternal safety. Traditionally, the gestational age at which treatment is deferred until fetal maturity is achieved is 20 weeks; prior to 20 weeks, treatment may commence with the fetus in utero. Because of improvements in neonatal survival with antenatal steroids and artificial surfactant, some clinicians have suggested that the gestational age at which treatment is deferred be moved back to 16 weeks.203 Regardless, treatment of early-stage carcinoma of the cervix is essentially the same as when there is no pregnancy, although surgery is preferred. Patients with early-stage disease can be treated with a radical hysterectomy. The operation is complicated by greater average blood loss,204 but made easier by the fact that planes of dissection are frequently more easily identified. If the fetus has reached viability, the operation should be preceded by a classic cesarean section. Survival is comparable with that of nonpregnant patients matched for stage of disease.205, 206
In non-pregnant patients desiring future fertility, a radical trachelectomy may be considered if certain criteria are met. Pathology should be carefully reviewed and confirmed to be squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Patients with stage Ia1 disease with lymphovascular space invasion, stage Ia2 disease, or stage Ib1 disease may qualify for this surgical approach. The tumor must be confined to the cervix and ideally less than 2 cm. Imaging studies including chest radiography and pelvic MRI should be negative for metastases. There should not be any evidence of prior infertility.207 The radical trachelectomy may be accomplished through a vaginal or abdominal approach. The oncologic outcomes of women with stage Ib1 cervical carcinoma undergoing radical trachelectomy appear comparable to a similar group of patients who had a radical hysterectomy.208 A recent review reported that after undergoing a radical trachelectomy, approximately 70% of women who attempted pregnancy were successful. Of these, 50% gave birth after 36 weeks, 21% gave birth in the third trimester prior to 36 weeks, 8% miscarried in the second trimester, and 21% had a miscarriage in the first trimester.209 Recent studies have also evaluated the use of robotic radical trachelectomy. A retrospective study by Nick et al. compared 25 patients who underwent attempted open radical trachelectomy to 12 patients that underwent attempted robotic radical trachelectomy. Robotic trachelectomy was associated with decreased blood loss (62.5 mL vs. 300 mL, p = 0.0001) and shorter postoperative stay (1 vs. 4 days, p <0.001), with no difference in operative time or histopathologic outcomes, indicating that this may be a promising and minimally invasive approach to fertility sparing surgery in the future.210
REFERENCES
Clark JG: A more radical method of performing hysterectomy for cancer of the uterus. Bull Johns Hopkins Hosp 6: 120, 1895 |
|
Rumpf H: Sitzung der Berliner Gesselschaff. Geb u Gyn Centr f Gyn 31: 849, 1895 |
|
Ries E: Eine neue Operationsmethode des uteruscarcinomas. Z Geburtshilfe Gynakol Stuttgart 37: 518, 1897 |
|
Schauta F: Die operation des gebarmutterkrebes mittels des Schuchardt schen paravaginalschnittes. Montasschr Z Geburtschilfe Gynakol 15: 133, 1902 |
|
Wertheim E: Zur frage der radikaloperation beim uteruskebs. Arch Gynecol 61: 627, 1900 |
|
Wertheim E: Discussion on the diagnosis and treatment of carcinoma of the uterus. BMJ 2: 689, 1905 |
|
Wertheim E: The extended abdominal operation for carcinoma uteri. Translated by Grad H Am J Obstet Dis Women Child 66: 169, 1912 |
|
Taussig FJ: Iliac lymphadenectomy with irradiation in the treatment of cancer of the cervix. Am J Obstet Gynecol 28: 650, 1934 |
|
Meigs JV: Carcinoma of the cervix: The Wertheim operation. Surg Gynecol Obstet 78: 195, 1944 |
|
Meigs JV: The Wertheim operation for carcinoma of the cervix. Am J Obstet Gynecol 49: 542, 1945 |
|
Meigs JV: Radical hysterectomy with bilateral pelvic lymph node dissections. Report of 100 cases operated on 5 years or more Am J Obstet Gynecol 62: 854, 1951 |
|
Lawton FG, Hacker NF: Surgery for invasive gynecologic cancer in the elderly female population. Obstet Gynecol 76: 287, 1990 |
|
Geisler JP, Geisler HE: Radical hysterectomy in the elderly female: A comparison to patients age 50 or younger. Gynecol Oncol 80: 258, 2001 |
|
Levrant SG, Fruchter RG, Maiman M: Radical hysterectomy for cervical cancer: Morbidity and survival in relation to weight and age. Gynecol Oncol 45: 317, 1992 |
|
Mousavi A, Karimi Zarchi M, Gilani MM et al: Radical hysterectomy in the elderly. World J Surg Oncol 6: 38, 2008 |
|
Soisson AP, Soper JT, Berchuck A, et al: Radical hysterectomy in obese women. Obstet Gynecol 80: 940, 1992 |
|
Cohn DE, Swisher EM, Herzog TJ, et al: Radical hysterectomy for cervical cancer in obese women. Obstet Gynecol 96: 727, 2000 |
|
Frumovitz M, Sun CC, Jhingran A et al: Radical hysterectomy in obese and morbidly obese women with cervical cancer. Obstet Gynecol 112: 899–905, 2008 |
|
Sutton GP, Bundy BN, Delgado G, et al: Ovarian metastases in stage Ib carcinoma of the cervix: A Gynecologic Oncology Group study. Am J Obstet Gynecol 166: 50, 1992 |
|
Bouma J, Hollema H: Case report: Ovarian metastasis from squamocellular cervical cancer, stage Ib and IIa. Acta Obstet Gynecol Scand 68: 471, 1989 |
|
Landoni F, Zanagnolo V, Lovato-Diaz L et al: Ovarian metastases in early-stage cervical cancer (IA2-IIA): a multicenter retrospective study of 1965 patients (a Cooperative Task Force study). Int J Gynecol Cancer 17: 623–8, 2007 |
|
Mann WJ, Chumas J, Amalfitano T, et al: Ovarian metastases from stage Ib adenocarcinoma of the cervix. Cancer 60: 1123, 1987 |
|
Natsume N, Aoki Y, Kase H, et al: Ovarian metastasis in stage Ib and II cervical adenocarcinoma. Gynecol Oncol 74: 255, 1999 |
|
Husseinzadeh N, Van Aken ML, Aron B: Ovarian transposition in young patients with invasive cervical cancer receiving radiation therapy. Int J Gynecol Cancer 4: 61, 1994 |
|
Husseinzadeh N: The preservation of ovarian function in young women undergoing pelvic radiation therapy. Gynecol Oncol 18: 373, 1984 |
|
Morice P, Juncker L, Rey A, et al: Ovarian transposition for patients with cervical carcinoma treated by radiosurgical combination. Fertil Steril 74: 743, 2000 |
|
Abitbol MM, Davenport JH: Sexual dysfunction after therapy for cervical carcinoma. Am J Obstet Gynecol 119: 181, 1974 |
|
Bergmark K, Avall-Lundqvist E, Dickman PW, et al: Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med 340: 1983, 1999 |
|
Schover L, Fife M, Gershensen DM: Sexual dysfunction and treatment for early stage cervical cancer. Cancer 63: 204, 1989 |
|
Lamb MA: Psychosexual issues: The woman with gynecologic cancer. Semin Oncol Nurs 6: 237, 1990 |
|
Anderson BL, Woods XA, Copeland LJ: Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 65: 221, 1997 |
|
Jensen PT, Groenvold M, Klee MC et al: Early-stage cervical carcinoma, radical hysterectomy, and sexual function. A longitudinal study. Cancer 100: 97–106, 2004 |
|
Frumovitz M, Sun CC, Schover LR et al: Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol 23: 7428–36, 2005 |
|
Lagasse LD, Creasman WT, Shingleton HM, et al: Results and complications of operative staging in cervical cancer: experience of the Gynecologic Oncology Group. Gynecol Oncol 9: 90, 1980 |
|
La Polla JP, Schlaerth JB, Gaddis O, et al: The influence of surgical staging on the evaluation and treatment of patients with cervical carcinoma. Gynecol Oncol 24: 194, 1986. |
|
Lanciano RM, Won M, Hanks GE: A reappraisal of the International Federation of Gynecologic and Obstetrics staging system for cervical cancer. A study of patterns of care Cancer 69: 482, 1992 |
|
Shingleton HM, Orr JW: Diagnosis, staging, and selectionof therapy for invasive tumors. Cancer of the Cervix. pp 108, 109 Philadelphia, Lippincott, 1995 |
|
Camilien L, Fordon D, Fruchter RG, et al: Predictive value of computerized tomography in the presurgical evaluation of primary carcinoma of the cervix. Gynecol Oncol 30: 209, 1988 |
|
Heller PB, Malfatano JH, Bundy BN: Clinical pathologic study of stages IIb, III, and IVa carcinoma of the cervix: Extended diagnostic evaluation for paraaortic node metastasis (a GOG study). Gynecol Oncol 38: 425, 1990 |
|
Russell AH, Shingleton HM, Jones WB, et al: Diagnostic assessment in patients with invasive cancer of the cervix: A national Patterns of Care study of the American College of Surgeons. Gynecol Oncol 63: 159, 1996 |
|
Zornoza J, Lukeman JM, Jing BS, et al: Percutaneous retroperitoneal lymph node biopsy in carcinoma of the cervix. Gynecol Oncol 5: 43, 1977 |
|
Hricak J, Lacey CG, Sandles LG, et al: Invasive cervical carcinoma: Comparison of MR imaging and surgical findings. Radiology 166: 623, 1988 |
|
Kolbenstvedt A, Knudson OS: A method for lymphangiographic and histologic correlation: Experience from 300 patients treated by pelvic lymphadenectomy. Gynecol Oncol 2: 9, 1974 |
|
Lagasse LD, Ballon SC, Berman ML, et al: Pretreatment lymphangiography and operative evaluation in carcinoma of the cervix. Am J Obstet Gynecol 134: 219, 1979 |
|
Rose PG, Adler LP, Rodriguez M, et al: Positron emission tomography for evaluating paraaortic nodal metastases in locally advanced cervical cancer before surgical staging: A surgicopathologic study. J Clin Oncol 17: 41, 1999 |
|
Narayan K, Hicks RJ, Jobling T, et al: A comparison of MRI and PET scanning in surgically staged loco-regionally advanced cervical cancer: Potential impact on treatment. Int J Gynecol Cancer 11: 263, 2001 |
|
Mitchell DG, Snyder B, Coakley F et al: Early invasive cervical cancer: tumor delineation by magnetic resonance imaging,computed tomography, and clinical examination, verified by pathologic results, inthe ACRIN 6651/GOG 183 Intergroup Study. J Clin Oncol 24: 5687–94, 2006 |
|
Schellas HF: Extraperitoneal paraaortic node dissection through an upper abdominal incision. Obstet Gynecol 46: 444, 1975 |
|
Berman ML, Lagasse LD, Watring WG, et al: The operative evaluation of patients with cervical carcinoma by an extraperitoneal approach. Obstet Gynecol 50: 658, 1977 |
|
Querleu D, Leblanc E, Castelain B: Laparoscopic pelvic lymphadenectomy in the staging of early carcinoma of the cervix. Am J Obstet Gynecol 164: 579, 1991 |
|
Heaps JM, Berek JS: Surgical staging of cervical cancer. Clin Obstet Gynecol 33: 852, 1990 |
|
Frumovitz M, Ramirez PT, Levenback CF: Lymphatic mapping and sentinel lymph node detection in women with cervical cancer. Gynecol Oncol 110 (3 Suppl 2): S17–20, 2008 |
|
Yuan SH, Xiong Y, Wei M et al: Sentinel lymph node detection using methylene blue in patients with early stagecervical cancer. Gynecol Oncol 106: 147–52, 2007 |
|
Silva LB, Silva-Filho AL, Traiman P et al: Sentinel node detection in cervical cancer with (99m)Tc-phytate. Gynecol Oncol 97: 588–95, 2005 |
|
Coutant C, Morel O, Delpech Y et al: Laparoscopic sentinel node biopsy in cervical cancer using a combined detection: Ann Surg Oncol 14: 2392–9, 2007 |
|
Malur S, Krause N, Kohler C et al: Sentinel lymph node detection in patients with cervical cancer. Gynecol Oncol 80: 254–7, 2001 |
|
Frumovitz M, Coleman RL, Gayed IW et al: Usefulness of preoperative lymphoscintigraphy in patients who undergo radical hysterectomy and pelvic lymphadenectomy for cervical cancer. Am J Obstet Gynecol 194: 1186–93; discussion 1193–5, 2006 |
|
Piver MS, Rutledge FN, Smith JP: Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol 44: 265, 1974 |
|
Okabayashi H: Radical abdominal hysterectomy for cancer for the cervix uteri. Surg Gynecol Obstet 33: 335, 1921 |
|
Shingleton HM, Gusberg SB: Radical hysterectomy. In Gusberg SB, Shingleton HM, Deppe G (eds): Female Genital Cancer. p 535, New York, Churchill Livingstone, 1988 |
|
Hacker NF: Cervical Cancer. In Berek JS, Hacker NF (eds): Practical Gynecologic Oncology. p 345, Philadelphia, Lippincott, Williams & Wilkins, 2000 |
|
Invasive Cervical Cancer. In Disaia PJ, Creasman WT (eds): Clinical Gynecologic Oncology. p 51, St. Louis, Mosby, 1997 |
|
Wheeles CR: Atlas of Pelvic Surgery. p 423, Baltimore, Williams & Wilkins, 1997 |
|
Averette HE, Method MW, Sevin BU, et al: Radical abdominal hysterectomy in the primary management of invasive cervical cancer. In Rubin SC, Hoskins WJ (eds): Cervical Cancer and Preinvasive Neoplasia. p 189, Philadelphia, Lippincott-Raven, 1996 |
|
Frumovitz M, dos Reis R, Sun CC et al: Comparison of total laparoscopic and abdominal radical hysterectomy for patients Obstet Gynecol 110: 96–102, 2007 |
|
Johnson N, Barlow D, Lethaby A et al: Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev (1): CD003677, 2005 |
|
Magrina JF, Kho RM, Weaver AL et al: Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol 109: 86–91, 2008 |
|
Fanning J, Fenton B, Purohit M: Robotic radical hysterectomy. Am J Obstet Gynecol 198: 649.e1–4, 2008 |
|
Roche WO, Norris HC: Microinvasive carcinoma of the cervix. Cancer 36: 180, 1975 |
|
Burke TW, Hoskins WJ, Heller PB, et al: Prognostic factors associated with radical hysterectomy failure. Gynecol Oncol 48: 571, 1976 |
|
Leman M, Benson W, Kurman R, et al: Microinvasive carcinoma of the cervix. Obstet Gynecol 48: 571, 1976 |
|
Seski JC, Abell MR, Morley GW: Microinvasive squamous cell carcinoma of the cervix. Definition, histologic analysis, late results of treatment Obstet Gynecol 50: 410, 1977 |
|
van Nagell JR, Donaldson ES, Wood EG, et al: The significance of vascular invasion and lymphocytic infiltration in invasive cervical cancer. Cancer 41: 228, 1978 |
|
Boyce JG, Fruchter RG, Nicastri AD, et al: Prognostic factors in stage I carcinoma of the cervix. Gynecol Oncol 12: 154, 1981 |
|
Larsson G, Alm P, Gullberg B, et al: Prognostic factors associated with radical hysterectomy failure. Gynecol Oncol 146: 145, 1983 |
|
Nahhas WA, Sharkey FE, Whitney CW, et al: The prognostic significance of vascular channel involvement and deep stromal penetration in early cervical carcinoma. Am J Clin Oncol 6: 259, 1983 |
|
Noguchi H, Shiozawa K, Tsukamoto T, et al: The postoperative classification for uterine cancer and its clinical evaluation. Gynecol Oncol 16: 219, 1983 |
|
Boyce JG, Fruchter RG, Nicastri AD, et al: Vascular invasion in stage I carcinoma of the cervix. Cancer 53: 1175, 1984 |
|
Matsuyama T, Inoue I, Tsukamoto N, et al: Stage Ib, IIa, and IIb cervix cancer, postsurgical staging, and prognosis. Cancer 54: 3072, 1984 |
|
White CD, Morley GW, Kumar ND: The prognostic significance of tumor emboli in lymphatic or vascular spaces of the cervical stroma in stage Ib squamous cell carcinoma of the cervix. Am J Obstet Gynecol 149: 342, 1984 |
|
Gauthier P, Gore H, Shingleton HM, et al: Identification of histopathologic risk groups in early-stage cervical cancer. Obstet Gynecol 66: 569, 1985 |
|
Creasman WT, Soper JT, Clarke-Pearson D: Radical hysterectomy as therapy for early carcinoma of the cervix. Am J Obstet Gynecol 155: 964, 1986 |
|
Simon NL, Gore H, Shingleton HM, et al: Study of superficially invasive carcinoma of the cervix. Obstet Gynecol 68: 19, 1986 |
|
Lim CS, Alexander-Sefre F, Allam M et al: Clinical value of immunohistochemically detected lymphovascular space invasion inearly stage cervical carcinoma. Ann Surg Oncol 15: 2581–8, 2008 |
|
Marchiole P, Buenerd A, Benchaib M et al: Clinical significance of lympho vascular space involvement and lymph node Gynecol Oncol 97: 727–32, 2005 |
|
Creasman WT, Kohler MF: Is lymph vascular space involvement an independent prognostic factor in early cervical cancer? Gynecol Oncol 92: 525–9, 2004 |
|
Christopherson WM, Gray LA, Parker JE: Microinvasive carcinoma of the uterine cervix. Cancer 38: 629, 1976 |
|
Ruch RM, Pitoch JA, Ruch WAJ: Microinvasive carcinoma of the cervix. Am J Obstet Gynecol 125: 87, 1976 |
|
Hasumi K, Sakamoto A, Sugano H: Microinvasive carcinoma of the uterine cervix. Cancer 45: 928, 1980 |
|
van Nagell JR, Greenwell N, Powell DF, et al: Microinvasive carcinoma of the cervix. Am J Obstet Gynecol 145: 981, 1983 |
|
Creasman WT, Fetter BF, Clarke-Pearson DL, et al: Management of stage Ia carcinoma of the cervix. Am J Obstet Gynecol 153: 164, 1985 |
|
Maiman MA, Fruchter RG, DiMaio TM, et al: Superficially invasive squamous cell carcinoma of the cervix. Obstet Gynecol 72: 399, 1988 |
|
Kolstad P: Follow-up study of 232 patients with stage Ia1 and 411 patients with stage Ia2 squamous cell carcinoma of the cervix (microinvasive carcinoma). Gynecol Oncol 48: 125, 1989 |
|
Tsukamoto N, Kaku T, Matsukuma K, et al: The problem of stage Ia (FIGO, 1985) carcinoma of the uterine cervix. Gynecol Oncol 34: 1, 1989 |
|
Ostor AG: Pandora's box or Ariadne's thread? Definition and prognostic significance of microinvasion in the uterine cervix: Squamous lesions Pathology Annual. p 103, Part II. Melbourne, Department of Pathology, 1995 |
|
Buckley SL, Tritz DM, van Le L, et al: Lymph node metastases and prognosis in patients with stage Ia2 cervical cancer. Gynecol Oncol 63: 4, 1996 |
|
Roman LD, Felix JC, Muderspach LI, et al: Risk of residual invasive disease in women with microinvasive squamous cancer in a conization specimen. Obstet Gynecol 90: 759, 1997 |
|
Creasman WT, Zaino RJ, Major FJ, et al: Early invasive carcinoma of the cervix (3 to 5 mm invasion): risk factors and prognosis. A GOG study Am J Obstet Gynecol 178: 62, 1998 |
|
Covens A, Shaw P, Murphy J, et al: Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage Ia-b carcinoma of the cervix? Cancer 86: 2273, 1999 |
|
Liu W, Meigs JV: Radical hysterectomy and pelvic lymphadenectomy: A review of 473 cases including 244 for primary invasive cancer of the cervix. Am J Obstet Gynecol 69: 1, 1955 |
|
Roddick JW, Greenlaw RH: Treatment of cervical cancer. A randomized study of operation and radiation Am J Obstet Gynecol 119: 754, 1971 |
|
Park RC, Patow WE, Rogers RR et al: Treatment for stage I carcinoma of the cervix. Obstet Gynecol 41: 117, 1973 |
|
Newton M: Radical hysterectomy or radiotherapy for stage I cervical cancer. A prospective comparison with 5 and 10 years follow-up Am J Obstet Gynecol 123: 535, 1975 |
|
Hoskins WJ, Ford JH, Lutz MH, et al: Radical hysterectomy and pelvic lymphadenectomy for the management of early invasive cancer of the cervix. Am J Obstet Gynecol 4: 278, 1976 |
|
Morley GW, Seski JC: Radical pelvic surgery versus radiation therapy for stage I carcinoma of the cervix (exclusive of microinvasion). Am J Obstet Gynecol 126: 785, 1976 |
|
Delgado G: Stage Ib squamous cell cancer of the cervix: The choice of treatment. Obstet Gynecol Surv 33: 174, 1978 |
|
Sall S, Pineda AA, Calanog A, et al: Surgical treatment of stages Ib and IIa invasive carcinoma of the cervix by radical abdominal hysterectomy. Am J Obstet Gynecol 135: 442, 1979 |
|
Langley I, Moore DW, Tarnasky J, et al: Radical hysterectomy and pelvic node dissection. Gynecol Oncol 9: 37, 1980 |
|
Benedet JL, Turko M, Boyes DA, et al: Radical hysterectomy in the treatment of cervical cancer. Am J Obstet Gynecol 137: 254, 1980 |
|
Webb MJ, Symmonds RE: Wertheim hysterectomy: A reappraisal. Obstet Gynecol 54: 140, 1979 |
|
Zander J, Baltzer J, Lohe KJ, et al: Carcinoma of the cervix: An attempt to individualize treatment; results of a 20-year cooperative study. Am J Obstet Gynecol 139: 752, 1981 |
|
Powell JL, Burrell MO, Franklin EW: Radical hysterectomy and pelvic lymphadenectomy. South Med J 77: 596, 1984 |
|
Artman LE, Hoskins WJ, Bibro MC, et al: Radical hysterectomy and pelvic lymphadenectomy for stage Ib carcinoma of the cervix: 21 years' experience. Gynecol Oncol 28: 8, 1987 |
|
Burghardt E, Pickel H, Hass J, et al: Prognostic factors and operative treatment of stages Ib to IIb cervical cancer. Am J Obstet Gynecol 156: 988, 1987 |
|
Bianchi UA, Sartori E, Pecorelli S, et al: Treatment of primary invasive cervical cancer. Considerations on 997 consecutive cases Eur J Gynaecol Oncol 9: 47, 1988 |
|
Kenter GG, Ansink AG, Heintz APM, et al: Carcinoma of the uterine cervix stage Ib and IIa: Results of surgical treatment: Complications, recurrence and survival. Eur J Surg Oncol 15: 55, 1989 |
|
Lee YN, Wang KL, Lin CH, et al: Radical hysterectomy with pelvic lymph node dissection for treatment of cervical cancer: A clinical review of 954 cases. Gynecol Oncol 32: 135, 1989 |
|
Monaghan JM, Ireland D, Shlomo MY, et al: Role of centralization of surgery in stage Ib carcinoma of the cervix: A review of 498 cases. Gynecol Oncol 37: 206, 1990 |
|
Ayhan A, Tuncer ZS: Radical hysterectomy with lymphadenectomy for treatment of early stage cervical cancer: Clinical experience of 278 cases. J Surg Oncol 47: 175, 1991 |
|
Alvarez RD, Potter ME, Soong S, et al: Rationale for using pathologic tumor dimensions and nodal status to subclassify surgically treated stage Ib cervical cancer patients. Gynecol Oncol 43: 108, 1991 |
|
Hopkins MP, Morley GW: Radical hysterectomy versus radiation therapy for stage Ib squamous cell cancer of the cervix. Cancer 68: 272, 1991 |
|
Averette HE, Nguyen HN, Donato DM, et al: Radical hysterectomy for invasive cervical cancer: A 25-year prospective experience with the Miami technique. Cancer 71: 1422, 1993 |
|
Landoni F, Maneo A, Colombo A, et al: Randomized study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 350: 535, 1997 |
|
Samlal RA, van der Velden J, Ten Kate FJW, et al: Surgical pathologic factors that predict recurrence in stage Ib and IIa cervical carcinoma patients with negative pelvic nodes. Cancer 80: 1234, 1997 |
|
Covens A, Rosen B, Murphy J et al. How important is re momval of the parametrium at surgery for carcinoma of the cervix? Gynecol Oncol 2002; 84 (1): 145-9. |
|
Steed H, Capstick V, Schepansky A et al. Early cervical cancer and parametrial involvement: is it significant? Gynecol Oncol 2006; 103 (1): 53-7. |
|
Wright JD, Grigsby PW, Brooks R et al. Utility of parametrectomy for early stage cervical cancer treated with radical hysterectomy. Cancer 2007; 110: 1281-6. |
|
Rob L, Pluta M, Strnad P et al. A less radical treatment option to the fertility-sparing radical trachelectomy in patients with stage I cervical cancer. Gynecol Oncol. 2008 Nov;111(2 Suppl):S116-20. |
|
Ditto A, Solima E, Hanozet F et al. Ultraconservative fertility-sparing surgery in early cervical cancer. Gynecol Surg 2009; 6 (Suppl 1): S1-S31. |
|
f. Maneo A, Sideri M, Scambia G et al. Simple conization and lymphadenectomy for the conservative treatment of stage IB1 cervical cancer. An Italian experience. Gynecol Oncol. 2011 Dec;123(3):557-60. |
|
Piver MS, Chung WS: Prognostic significance of cervical lesion size and pelvic node metastases in cervical carcinoma. Obstet Gynecol 46: 507, 1975 |
|
Fuller AF, Elliot N, Kosloff C, et al: Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage Ib and IIa carcinoma of the cervix. Gynecol Oncol 33: 34, 1989 |
|
Rettenmaier MA, Casanova DM, Micha JP, et al: Radical hysterectomy and tailored postoperative radiation therapy in the management of bulky stage Ib cervical cancer. Cancer 63: 2220, 1989 |
|
Delgado G, Bundy B, Zaino R, et al: Prospective surgical-pathological study of disease-free interval in patients with stage Ib squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol Oncol 38: 352, 1990 |
|
Bloss JD, Berman ML, Murherjee J, et al: Bulky stage Ib cervical carcinoma managed by primary radical hysterectomy followed by tailored radiotherapy. Gynecol Oncol 47: 21, 1992 |
|
Alvarez RD, Gelder MS, Gore H, et al: Radical hysterectomy in the treatment of patients with bulky early-stage carcinoma of the cervix uteri. Surg Gynecol Obstet 176: 539, 1993 |
|
Ho CM, Chien TY, Huang SH et al: Multivariate analysis of the prognostic factors and outcomes in early cervicalcancer patients undergoing radical hysterectomy. Gynecol Oncol 93: 458–64, 2004 |
|
Fletcher G, Hamberger A: Squamous cell carcinoma of the uterine cervix. In Fletcher G (ed): Textbook of Radiotherapy. p 720, Philadelphia, Lea & Febiger, 1980 |
|
Montana GS, Fowler WC, Varia MA, et al: Carcinoma of the cervix stage Ib; results of treatment with radiation therapy. Int J Radiat Oncol Biol Phys 9: 45, 1983 |
|
Perez CA, Grigsby PW, Nene SW, et al: Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer 69: 2796, 1991 |
|
Lowrey GC, Mendenhall WM, Million RR: Stage Ib or IIa-b carcinoma of the intact uterine cervix treated with irradiation; a multivariate analysis. Int J Radiat Oncol Biol Phys 24: 205, 1992 |
|
Finan MA, DeCesare S, Fiorca JV, et al: Radical hysterectomy for stage Ib1 vs Ib2 carcinoma of the cervix: Does the new staging system predict morbidity and survival? Gynecol Oncol 62: 139, 1996 |
|
Rutledge TL, Kamelle SA, Tillmanns TD et al: A comparison of stages IB1 and IB2 cervical cancers treated with radicalhysterectomy. Is size the real difference? Gynecol Oncol 95: 70–6, 2004 |
|
Sedlis A, Bundy BN, Rotman MZ, et al: A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage Ib carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group study. Gynecol Oncol 73: 177, 1999 |
|
van der Velden J, Samlal R, Schilthuis MS, et al: A limited role for adjuvant radiotherapy after the Wertheim/Okabayashi Radical Hysterectomy for cervical cancer confined to the cervix. Gynecol Oncol 75: 233, 1999 |
|
Rotman M, Sedlis A, Piedmonte MR et al: A phase III randomized trial of postoperative pelvic irradiation in Stage IB oncology group study. Int J Radiat Oncol Biol Phys 65: 169–76, 2006 |
|
Landoni F, Maneo A, Colombo A et al: Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 350: 535–40, 1997 |
|
Keys HM, Bundy BN, Stehman FB et al: Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 340: 1154–61, 1999 |
|
Morris M, Eifel PJ, Lu J et al: Pelvic radiation with concurrent chemotherapy compared with pelvic and N Engl J Med 340: 1137–43, 1999 |
|
Peters WA 3rd, Liu PY, Barrett RJ 2nd et al: Concurrent chemotherapy and pelvic radiation therapy compared with pelvic J Clin Oncol 18: 1606–13, 2000 |
|
Rocconi RP, Estes JM, Leath CA 3rd et al: Management strategies for stage IB2 cervical cancer: a cost-effectiveness analysis. Gynecol Oncol 97: 387–94, 2005 |
|
Jewell EL, Kulasingam S, Myers ER et al: Primary surgery versus chemoradiation in the treatment of IB2 cervical carcinoma:a cost effectiveness analysis. Gynecol Oncol 107: 532-40, 2007 |
|
Eddy GL, Bundy BN, Creasman WT et al: Treatment of ("bulky") stage IB cervical cancer with or without neoadjuvant lymphadenectomy: a phase III trial of the gynecologic oncology group. Gynecol Oncol 106: 362–9, 2007 |
|
Coleman RL, et al: Radical hysterectomy for recurrent carcinoma of the uterine cervix after radiotherapy. Gynecol Oncol 55: 29, 1994 |
|
Rutledge S, Carey MS, Pritchard J, et al: Conservative surgery for recurrent or persistent carcinoma of the cervix following irradiation; is exenteration always necessary? Gynecol Oncol 52: 353, 1994 |
|
Maneo A, Landoni F, Cormio G, et al: Radical hysterectomy for recurrent or persistent cervical cancer following radiation therapy. Int J Gynecol Cancer 9: 295, 1999 |
|
Lerner HM, Jones HW, Hill EC: Radical surgery for the treatment of early invasive cervical carcinoma (stage Ib): Review of 5 years’ experience. Obstet Gynecol 56: 413, 1980 |
|
Samlal RAK, van der Velden J, Ketting BW, et al: Disease-free interval and recurrence pattern after the Okabayashi variant of Wertheim’s radical hysterectomy for stage Ib and IIa cervical carcinoma. Int J Gynecol Cancer 12: 23, 1981 |
|
Benjamin I, Barakat RR, Curtin JP, et al: Blood transfusion for radical hysterectomy before and after the discovery of transfusion-related human immunodeficiency virus infection. Obstet Gynecol 84: 97, 1994 |
|
Underwood PB, Wilson WC, Kreutner A, et al: Radical hysterectomy: A critical review of twenty-two years’ experience. Am J Obstet Gynecol 134: 889, 1979 |
|
Wheeles CR: The Gambee intestinal anastomosis in gynecologic surgery. Obstet Gynecol 46: 448, 1975 |
|
Powell JL, Burrell MO, Franklin EW: Radical hysterectomy and pelvic lymphadenectomy. Gynecol Oncol 12: 23, 1981 |
|
Sivanesaratnam V, Sen DK, Jayalakshmi P, et al: Radical hysterectomy and pelvic lymphadenectomy for early invasive cancer of the cervix: 14 years’ experience. Int J Gynecol Cancer 3: 231, 1993 |
|
Sevin BU, Ramos R, Lichtinger M, et al: Antibiotic prevention of infections complicating radical abdominal hysterectomy. Obstet Gynecol 64: 539, 1984 |
|
Clarke-Pearson DL, Jelovsek FR, Creasman WT: Thromboembolism complicating surgery for cervical and uterine malignancy: Incidence, risk factors, and prophylaxis. Obstet Gynecol 61: 87, 1983 |
|
Clarke-Pearson DL, Synan IS, Henshaw WM, et al: Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol 63: 92, 1984 |
|
Farquharson DIM, Orr JW: Thromboembolic complication in gynecology. J Reprod Med 29: 845, 1984 |
|
Merli GJ, Martinez J: Prophylaxis for deep vein thrombosis and pulmonary embolism in the surgical patient. Med Clin North Am 71: 377, 1987 |
|
Shingleton HM, Orr JW (eds): Cancer of the Cervix—Diagnosis and Treatment. p 132, New York, Churchill Livingstone, 1987 |
|
Seski JC, Dioknoac N: Bladder dysfunction after radical hysterectomy. Am J Obstet Gynecol 128: 6, 1977 |
|
Forney JP: The effect of radical hysterectomy on bladder physiology. Am J Obstet Gynecol 138: 374, 1980 |
|
Low JA, Mauger GM, Carmichael JA: The effect of Wertheim hysterectomy upon bladder and urethral function. Am J Obstet Gynecol 139: 826, 1981 |
|
Carenza L, Nobili F, Giacobini S: Voiding disorders after radical hysterectomy. Gynecol Oncol 13: 213, 1982 |
|
Petri E: Bladder after radical pelvic surgery. p 220, Staton SL (ed): Clinical Gynecologic Urology. St. Louis, CV Mosby, 1984 |
|
Westby M, Asmussen M: Anatomical and functional changes in the lower urinary tract after radical hysterectomy with lymph node dissection as studied by dynamic urethrocystography and simultaneous urethrocystometry. Gynecol Oncol 21: 261, 1985 |
|
Fishman IJ, Shbsigh R, Kaplan AL: Lower urinary tract dysfunction after radical abdominal hysterectomy for carcinoma of the cervix. Urology 28: 462, 1986 |
|
Farquharson DIM, Shingleton HM, Sanford SP, et al: The adverse effects of cervical cancer treatment on bladder function. Gynecol Oncol 27: 15, 1987 |
|
Ralph G, Tamussino K, Lichtenegger W: Urodynamics following abdominal hysterectomy for cervical cancer. Arch Gynecol Obstet 243: 215, 1988 |
|
Kristensen GB, Frimodt-Moller PC, Poulsen HK, et al: Persistent bladder dysfunction after surgical and combination therapy of cancer of the cervix uteri stages Ib and 2a. Gynecol Oncol 18: 38, 1984 |
|
Scotti RJ, Bergman A, Bhatia NN, et al: Urodynamic changes in ureterovesical function after radical hysterectomy. Obstet Gynecol 68: 111, 1986 |
|
Covens A, Rosen B, Gibbons A, et al: Differences in the morbidity of radical hysterectomy between gynecological oncologists. Gynecol Oncol 51: 39, 1993 |
|
Kadar N, Nelson JH: Treatment of urinary incontinence after radical hysterectomy. Obstet Gynecol 64: 400, 1984 |
|
Bandy LC, Clarke-Pearson GL, Soper JT, et al: Long-term effects on bladder function following radical hysterectomy with and without postoperative radiation. Gynecol Oncol 26: 160, 1987 |
|
Lin LY, Wu JH, Yang CW et al: Impact of radical hysterectomy for cervical cancer on urodynamic findings. Int Urogynecol J Pelvic Floor Dysfunct 15: 418–21, 2007 |
|
Larson DM, Malone JM, Copeland LJ, et al: Ureteral assessment after radical hysterectomy. Obstet Gynecol 69: 612, 1987 |
|
Ilancheran A, Monaghan JM: Pelvic lymphocysts—A 10-year experience. Gynecol Oncol 29: 333, 1988 |
|
Mann WJ, Vogel F, Patsner B, et al: Management of lymphocysts after radical gynecologic surgery. Gynecol Oncol 33: 248, 1989 |
|
Kikku P, Gronroos M, Taina E, et al: Colposcopic, cytological and histological evaluation of the cervical stump 3 years after supravaginal uterine amputation. Acta Obstet Gynecol Scand 64: 235, 1985 |
|
Miller BE, Copeland LJ, Hamberger AD, et al: Carcinoma of the cervical stump. Gynecol Oncol 18: 100, 1984 |
|
Porpora MG, Mobili F, Pietrangeli D, et al: Cervical stump carcinoma therapy. Eur J Gynaecol Oncol 12: 45, 1991 |
|
Petersen LK, Mamsen A, Jakobsen A: Carcinoma of the cervical stump. Gynecol Oncol 46: 199, 1992 |
|
Barillot I, Horiot JC, Cuisenier J, et al: Carcinoma of the cervical stump; a review of 213 cases. Eur J Cancer 29A: 1231, 1993 |
|
Orr JW, Ball GC, Soong SJ, et al: Surgical treatment of women found to have invasive cervix cancer at the time of total hysterectomy. Obstet Gynecol 68: 353, 1986 |
|
Kinney WK, Egorshin EV, Ballard DJ, et al: Long-term survival and sequelae after surgical management of invasive cervical carcinoma diagnosed at the time of simple hysterectomy. Gynecol Oncol 44: 24, 1992 |
|
Dargent D: A new future for Schauta’s operation through presurgical retroperitoneal pelvioscopy. Eur J Gynecol Oncol 8: 292, 1987 |
|
Querleu D: Laparoscopic pelvic lymphadenectomy in the staging of early carcinoma in the cervix. Am J Obstet Gynecol 164: 579, 1991 |
|
Daniel FG, Mathevet P: Radical vaginal hysterectomy in the primary treatment of invasive cervical cancer. In Rubin SC, Hoskins WJ (eds): Cervical Cancer and Preinvasive Neoplasia. pp 207, 217 Philadelphia, Lippincott-Raven, 1996 |
|
Burgess SP, Waymont B: Implantation of a cervical carcinoma in an episiotomy site. Case report Br J Obstet Gynaecol 94: 598, 1987 |
|
Copeland LJ, Saul PB, Sneige N: Cervical adenocarcinoma: tumor implantation in the episiotomy sites of two patients. Gynecol Oncol 28: 230, 1987 |
|
Gorvan AN, Jensen R, Jones HW: Squamous carcinoma of the cervix complicating pregnancy: Recurrence in episiotomy after vaginal delivery. Obstet Gynecol 73: 850, 1989 |
|
Cliby WA, Dodson MK, Podratz KC: Cervical cancer complicated by pregnancy: Episiotomy site recurrences following vaginal delivery. Obstet Gynecol 84: 179, 1994 |
|
Van Dam PA, Irvine L, Lowe DG, et al: Carcinoma in episiotomy scars. Gynecol Oncol 44: 96, 1992 |
|
Method MW, Brost BC: Management of cervical cancer in pregnancy. Semin Surg Oncol 16: 251, 1999 |
|
Monk BJ, Montz FJ: Invasive cervical cancer complicating intrauterine pregnancy: Treatment with radical hysterectomy. Obstet Gynecol 80: 199, 1992 |
|
van der Vange N, Weverling GJ, Ketting BW, et al: The prognosis of cervical cancer associated with pregnancy: A matched cohort study. Obstet Gynecol 85: 1022, 1995 |
|
Zemlickis D, Lishner M, Degendorfer P, et al: Maternal and fetal outcome after invasive cervical cancer in pregnancy. J Clin Oncol 9: 1956, 1991 |
|
Ramirez PT, Schmeler KM, Soliman PT et al: Fertility preservation in patients with early cervical cancer: radicaltrachelectomy. Gynecol Oncol 110 (3 Suppl 2): S25–8, 2008 |
|
Diaz JP, Sonoda Y, Leitao MM et al: Oncologic outcome of fertility-sparing radical trachelectomy versus radica lhysterectomy for stage IB1 cervical carcinoma. Gynecol Oncol 2008 [Epub ahead of print] |
|
Boss EA, van Golde RJ, Beerendonk CC et al: Pregnancy after radical trachelectomy: a real option? Gynecol Oncol 99 (3 Suppl 1): S152–6, 2005 |
|
Nick AM, Frumovitz MM, Soliman PT et al. Fertility sparing surgery for treatment of early-stage cervical cancer: Open vs. robotic radical trachelectomy. Gynecol Oncol. 2011 Oct 27. |